U.S. President Donald Trump says he has turned down a plan for White House officials to receive a coronavirus vaccine in the coming days.
Officials say senior members of the Trump administration will be among the first to receive the Pfizer / BioNTech jab.
Vaccine Rollout Has Begun
But Mr. Trump later wrote on Twitter that people working in the White House “should get this vaccine in time … unless necessary”.
The United States will begin rolling out the issue on Monday.
The vaccine provides up to 95% protection against Covid-19.
The first three million doses are still distributed in many locations in all 50 states throughout the United States. The first delivery of that dose left the area in Michigan on Sunday, with health workers and the elderly in line to get the first shot.
News on Sunday that White House workers will be among the first to be vaccinated has drawn criticism on social media. It is unclear why Mr. Trump decided to change these plans, or what the outcome would be in the government’s efforts to protect senior officials.
COVID Cases
Coronavirus mortality has risen sharply since November in the US, with a record daily rise of 3,309 reported on Saturday.
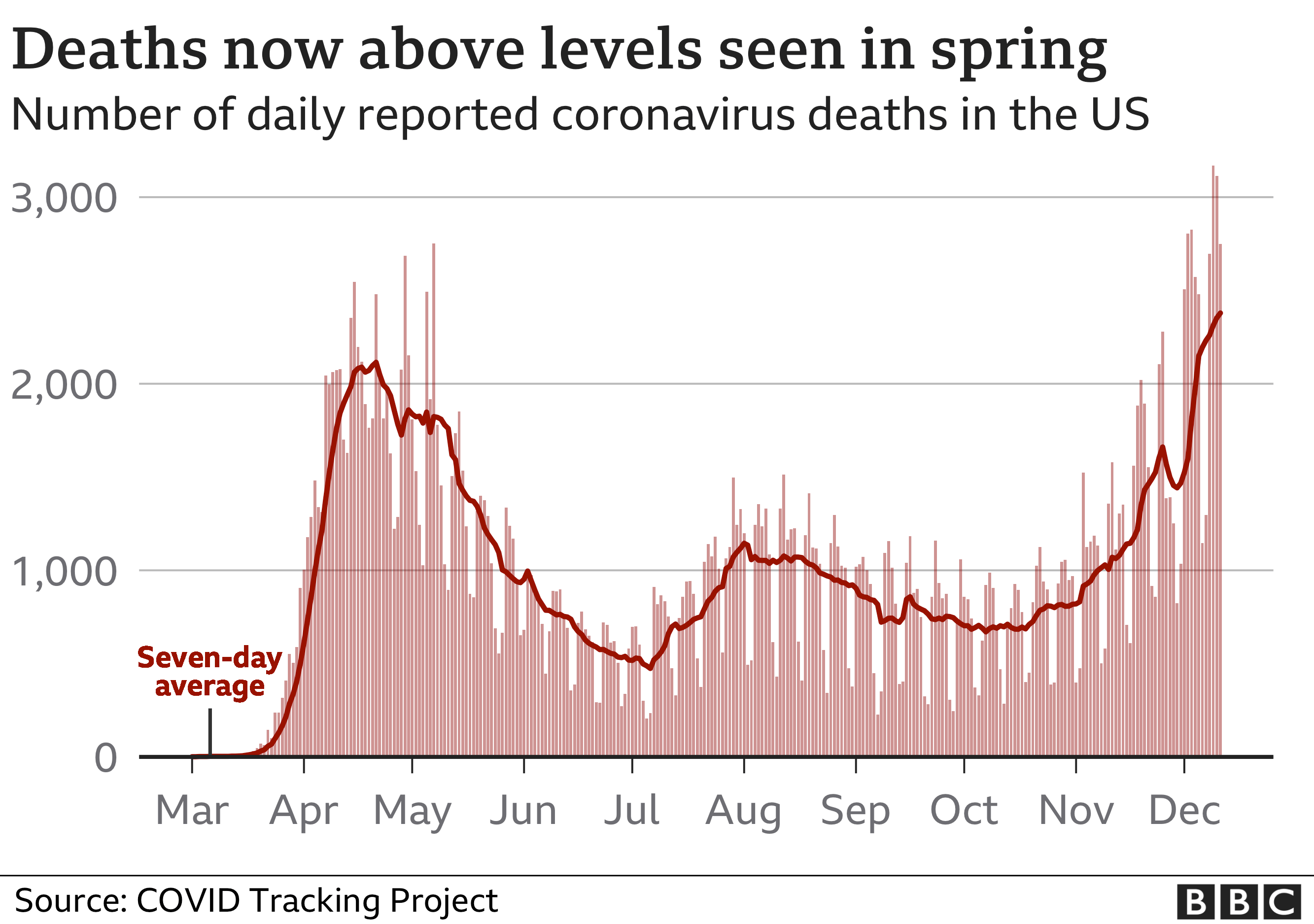
Distribution of the vaccine is set to change the course of the coronavirus epidemic, which has claimed the lives of nearly 300,000 people in the US.
Food and Drug Administration (FDA) officials say their approval for the use of emergency vaccines, announced on Friday, is “significant milestone” in the epidemic, after heavy pressure from Trump officials to approve the jab.
Doses of the same vaccine are already being treated in the UK. Pfizer vaccine has also been approved in Canada, Bahrain and Saudi Arabia as well.
The start of the US vaccination campaign comes as Electoral College – a US presidential election organization – is expected to approve Joe Biden’s victory on Monday.
When Will Trump Get The Vaccine?
Officials told several media outlets on Sunday that some of the vaccines would be reserved for those working close to Mr. Trump.
The vaccination program, first reported by the New York Times, was confirmed by National Security Council (NSC) spokesman John Ullyot.

One of the goals of the program was to build public confidence in the vaccine, he said.
“Americans need to be confident that they are getting a safe and effective vaccine like those in charge of the U.S. government through the advice of public health workers and national security leadership,” Mr Ullyot said.
But Mr Trump on Sunday suggested that top officials now wait a long time.
“People working in the White House should receive this injection later in the program, unless necessary,” he tweeted. “I have asked for this change to be made.”
The US president, who contracted coronavirus in October and recovered after being hospitalized, said he had not planned to take the vaccine but was looking forward to doing so “in due course.
He has previously claimed to have “immune system”, although medical experts say it is unclear whether people who received Covid-19 treatment were protected from second-line infections, and if so, how long could this protection last.
There has been a massive outbreak of coronavirus in the White House, with many senior staff and managers checking to be infected.
The latest was Trump’s lawyer, Rudy Giuliani, who revealed last week that he had been treated like a drug addict.
The Vaccine And Its Function
The Pfizer / BioNTech vaccine was the first jab of coronavirus to show promising results in the final stages of the testing process.
It is a new type called mRNA vaccine that uses a small piece of genetic code from the epidemic virus to teach the body how to fight Covid-19 and build up the immune system.
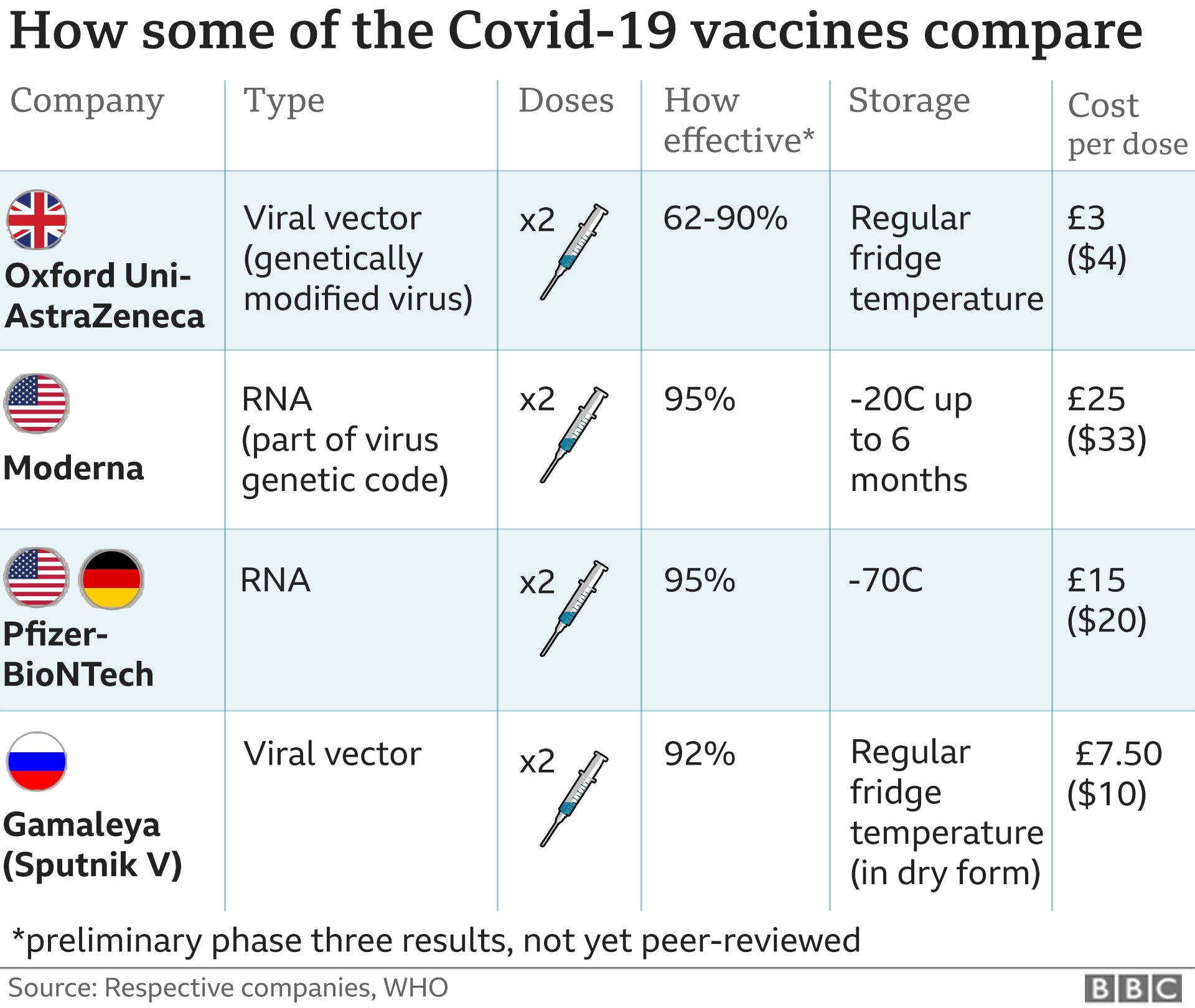
“The vaccine contains a small amount of mRNA virus [Covid-19] that directs cells in the body to make a different ‘spike’ protein for the virus,” the FDA said.
“When a person receives this vaccine, his or her body produces copies of spike protein, which do not cause the disease, but cause the immune system to learn the immune response, producing antibodies against [Covid-19].”
The vaccine is given in two injections, separated for 21 days, and the second dose is a stimulant. The immune system begins to enter after the first dose but reaches its full potential seven days after the second dose.
The vaccine should be stored at very low temperatures, making distribution difficult. Special shipping containers that use dry ice will be used to move frozen containers to the vaccine, Pfizer said.
The pharmaceutical company has agreed to offer the US 100 million doses of vaccine in March.
An additional 200 million doses of the second vaccine, developed by Moderna and the National Institutes of Health, will be released in June. However, the policy still requires approval in the US.






Leave a Reply
You must be logged in to post a comment.