 News
News
Shares of Covid-19 vaccine research company Novavax up 3500% – Here’s what you need to know
No one is unaware of the ongoing pandemic which has trapped us all under its wide shackles. But there are companies working towards potential vaccines and Novavax is one of them.
Novavax, Inc. is an American vaccine development company headquartered in Gaithersburg, Maryland, with additional facilities in Rockville, Maryland and Uppsala, Sweden. As of 2020, it has an ongoing Phase III clinical trial in older adults for its candidate vaccine for seasonal influenza, NanoFlu and a candidate vaccine for prevention of COVID-19.
The soaring price of Novavax’s shares
Shares of the biotech company were trading at less than $5 a share, and the coronavirus hadn’t yet embarked on its deadly trip throughout the world. Since then, the stock has increased nearly 34-fold, and Novavax has secured its spot among leaders in the race to develop a coronavirus vaccine.
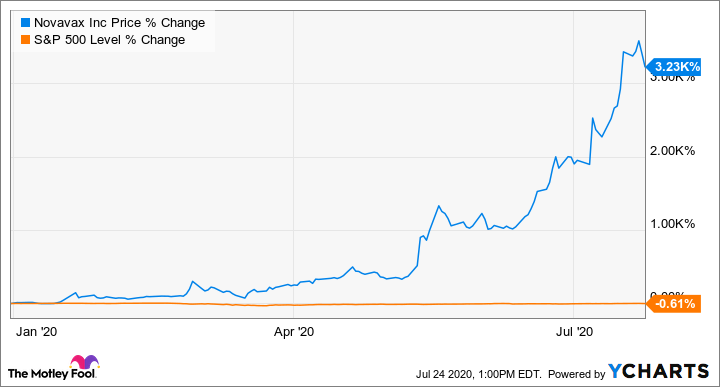
Novavax Inc.’s Covid-19 vaccine results are expected imminently and scientists will be scanning the data to gauge the shot’s promise. At the same time, day traders — who’ve snapped up shares since March — will be looking for the more than $7 billion surge in value to hold.
Vaccine candidates presented so far have detailed initial patient antibody and T-cell responses and compared them with levels seen in patients who have recovered from the virus. Investors will be looking for those initial immune-system responses when Novavax reveals its data, likely sometime next week.
Novavax is on its way to becoming a household name similar to Moderna Inc., another vaccine developer that has rallied since March but has yet to have a product reach the market. Recent $1.6 billion funding from the U.S. government has bolstered Novavax’s credibility despite some concern over its use of industry ties to get that backing.
The need for a vaccine is clear, with Covid-19 cases in the U.S. reaching beyond 4 million and the virus on the rise across the Sunbelt. There are roughly 25 vaccines in human testing with more than 140 others in earlier stages of development, according to the World Health Organization.
The market has responded positively to some of the first results in people from Moderna, as well as Pfizer Inc. and BioNTech SE’s messenger RNA vaccines. AstraZeneca Plc’s experimental shot with the University of Oxford was compared less favorably by some on Wall Street. Biotechs that didn’t provide sufficient detail have given back some of their share gains.
How is the vaccine being developed?
In January 2020, Novavax announced development of a vaccine candidate, named NVX-CoV2373, to establish immunity to SARS-CoV-2. NVX-CoV2373 is a protein subunit vaccine that contains the spike protein of the SARS-CoV-2 molecule. Novavax’s work is in competition for vaccine development among dozens of other companies.In March 2020, Novavax announced a collaboration with Emergent BioSolutions for preclinical and early-stage human research on the vaccine candidate. X-CoV2373 — uses insect cells to grow key proteins and the company has said it could be easier to mass produce than some rival shots. NVX-CoV2373 is being tested in a two dose regimen, with some patients getting a formulation enhanced with the company’s proprietary plant-based nanoparticle technology meant to further stimulate an immune response.

Results so far have measured these immune responses at different time points and using different measures, making them difficult to compare.
“It’s an imperfect situation,” said Steven Seedhouse, an analyst at Raymond James in a phone call. “There’s data out there, to the extent you can you do your best to interpret it.”
Early antibody results from BioNTech-partnered Pfizer have looked the most promising so far, said Seedhouse who has a doctorate in molecular pharmacology. “We don’t know what’s going to confer immunity and we certainly don’t know what’s going to confer durable immunity.”
Novavax’s Human Trial Phase
Novavax began enrolling participants in the phase 1 part of a phase 1/2 trial in May. The phase 2 portion will begin following phase 1 results on safety and immune response. Phase 1 is testing the vaccine on about 130 participants ages 18 to 59 in Australia. The second part of the trial will expand to more countries, including the U.S., and broaden the age range of volunteers. The first human safety studies of the candidate, named NVX-CoV2373, started in May 2020 in Australia.
A small side note on Novavax’s technology: The company doesn’t yet have products on the market, but it has proven the power of its vaccine development capabilities. Earlier this year, Novavax reported that its investigational flu vaccine candidate, NanoFlu, met all primary endpoints in a phase 3 trial. The company plans to submit the product to the U.S. Food and Drug Administration for review.
Also Read : News Highlights From July 2020
Recent Posts
- Pakistan Opens Doors to Boost Tourism and Business with Free Visas for 126 Countries
- Disney’s “Inside Out 2” Becomes the Highest-Grossing Animated Film Ever
- South Korea Cracks Down on Crypto Exchange Fees
- Ava Kris Tyson Departs MrBeast Following Grooming Allegations
- Apple’s iPhone SE 4: What do we know so far?
- ‘Inside Out 2’ Crushes Box Office and Enters the Billion-Dollar Club
- Telegram Founder Backs Viral Crypto Game Hamster Kombat, Calls it a New Era for Blockchain
- End of the Road for Self-Lacing Nikes: App Shutdown Leaves Adapt BB Owners with Limited Functionality
- Chaotic Encounter During European Tour Leaves IShowSpeed Injured and Frightened
- Quirky RPG ‘Thirsty Suitors’ Coming Soon to Mobile on Netflix Games


