 News
News
Rapid $25 At-Home COVID 19 Test Kit From Abbott Authorized By FDA
The Food and Drug Administration announced on Wednesday that it had approved Abbott Labs ’rapid testing of Covid-19 for home use, although doctors had to provide testing for patients.
15 Minute Quick Results
The test, which is an antigen test that produces results in about 15 minutes, was previously approved for use only by qualified staff, but the new permit will allow patients to test themselves at home with the help of a doctor.
It is the third U.S.-approved test. “Unusually used at home,” said Dr. Jeff Shuren, director of FDA’s Center for Devices and Radiological Health, in a statement.
How It Works?
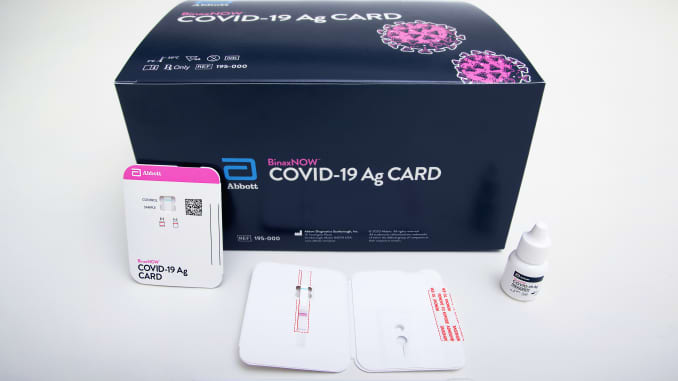
Dekivering the test, called BinaxNOW and costing $ 25 for home use, in private homes and overseeing the collection and testing process, Abbott said he has partnered with a telehealth provider at Med.
Patients collect the sample with the nasab swab themselves and the app helps guide the testing process and provide results, Abbott said.
Everyone 15 years and older who is suspected of having Covid-19 is their health care provider and within seven days of the onset of the disease is eligible for testing, the FDA has said.

The test can also be used for people 4 years or older, even if an adult needs to collect a sample, the agency said.
“The FDA continues to approve the COVID-19 trial which will give more Americans access to major testing options and options,” said FDA Commissioner Dr. Stephen Hahn in a statement. “The BinaxNOW COVID-19 Ag Home Test will have a virtual production facility capable of supporting the testing of millions of people.”
Other Approvals By The FDA
Abbott said that it expects to deliver 30 million home tests in the first quarter of 2021, and another 90 million in the second quarter. The FDA noted that antigen tests are not as accurate as most cellular tests.
“As the pandemic has progressed, the need for immediate testing has only increased. Unfortunately, we have heard that many people do not have access to the test as soon as they need it, ”Robert Ford, president and CEO of Abbott, said in a statement. “That’s why Abbott is bringing our fast BinaxNOW and NAVICA trial home.”
The FDA first approved the test to be used by trained staff in August, setting it up at that time as the first Covid-19 trial that cost about $ 5 and delivers results in minutes on a test card outside of laboratory equipment, such as a pregnancy test. The US quickly purchased $ 150 million $ 750 million tests to increase test capacity.
However, home use of the test will cost $ 25, more than $ 5 cost in medical facilities, Abbott said Wednesday.
“FDA approval of BinaxNOW card testing for home use means we must have tens of millions of COVID-19 tests in the coming months that Americans can use without leaving home,” Health and Human Services Secretary Alex Azar said in a statement Wednesday.
The approval comes after the FDA canceled Ellume’s Covid home test on Tuesday. The product is approved for use by anyone 2 years of age or older and does not require a prescription.
Synopsis
The Food and Drug Administration announced on Wednesday that it had approved Abbott Labs ’rapid testing of Covid-19 for home use, although doctors had to provide the prescriptions for patients.
It is the third U.S.-approved test. “Unusually used at home,” said Dr. Jeff Shuren, director of FDA’s Center for Devices and Radiological Health, in a statement.
Patients collect the sample with the nasal swab themselves and the app helps guide the testing process and provide results, Abbott said.
Recent Posts
- Feeling the Pressure at 25–30? Here’s Why Young Adults Think Time Is Running Out
- BTS Is Back! All Seven Members Complete Military Service—What’s Next for the K-Pop Kings?
- Diddy’s Blockbuster Trial Nears Verdict: What’s Next for the Hip-Hop Mogul?
- “Squid Game” Final Season Sparks Global Buzz Ahead of Premiere
- Beyoncé and Jay-Z Reunite Onstage in Paris: A Night to Remember
- Texas Bets Big on Film: $1.5 Billion Incentive Law Aims to Bring Hollywood Home
- Starbucks Considers Selling Its China Business Amid Fierce Local Competition
- JetBlue Retreats from Miami and Seattle Amid Mounting Financial Pressures
- Barbra Streisand Calls Out Hollywood’s Pay Gap in Meet the Fockers Revelation
- Elon Musk’s Robotaxi Revolution: A Cosmic Ride into the Future


