Modern vaccine is safe and 94% effective, say regulators, paving the way for US emergency approval.
Analysis by the Food and Drug Administration’s (FDA) means it could be the second approved coronavirus vaccine in the US.
Emergency Approval Application
It comes a day after Americans across the country began receiving Pfizer-BioNTech vaccine.
The news comes as the death toll from the American coronavirus exceeds 300,000, according to Johns Hopkins University.
The approval of the Modern vaccine vaccine by FDA scientists was announced on Tuesday, two days before the vaccination panel met to discuss emergency approval.
Over 90% Safety
The 54-page article said “there are no concerns about safety” and that serious side effects were rare.
If approved by a team of experts later this week, and by the FDA vaccine manager, submissions can begin within 24 hours.
The FDA received a 94.1% performance rating in a test of 30,000 people, according to a document.
Common side effects include fever, headache, and muscle and joint pain.
Last week, the FDA released similar data from Pfizer before voting to issue the permit.
Moderna was founded in 2010 and to date has never had a FDA-approved product.
The company’s stocks have seen an increase of about 700% so far this year.
Moderna Vs Pfizer – The Difference
Moderna vaccine requires temperatures around -20C for delivery – similar to a standard refrigerator.
Pfizer jab requires temperatures close to -75C, making transportation more difficult.

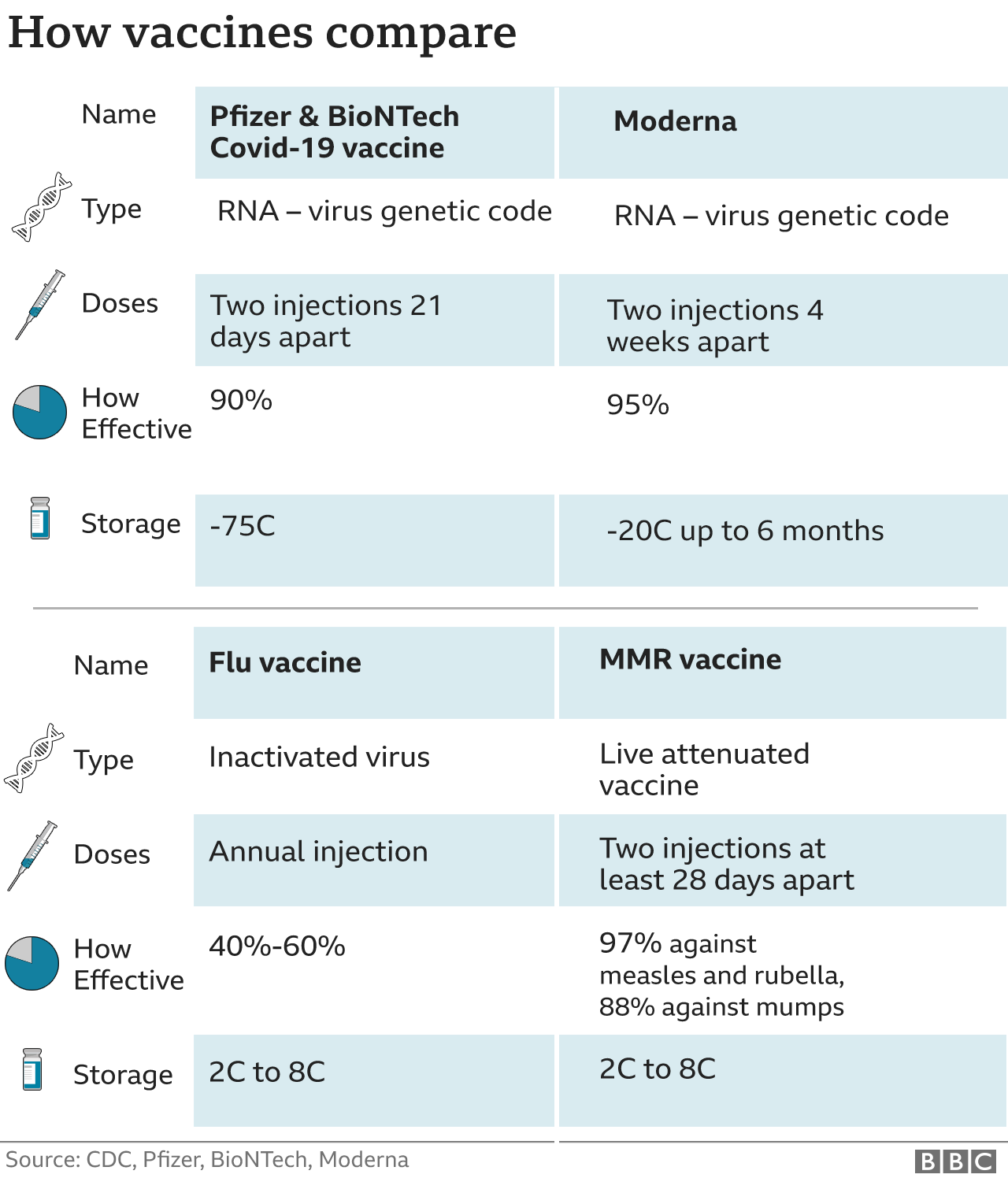
Like Pfizer jab, Moderna vaccine also requires a second supplement. Moderna’s second jab comes 28 days after the first.
The company is based in Cambridge, Massachusetts, and has said that if approved, “most of” its vaccine will be made there.
Pfizer’s drug is made in many countries, including Germany and Belgium.
Approval of the second vaccine against Covid in the US could be a big deal.
The country is the worst hit in the world according to the number of people who have died of the disease and we need immediate bullets to fight the virus.
The US has several million doses of the Pfizer-BioNTech Covid vaccine approved for emergency use a few days ago by regulators.
Although mass vaccination has already begun with this, gaining access to the Moderna jab soon, in addition to the extra stock of one Pfizer, could mean that millions of other people could be vaccinated in the coming months.
Food and Drug Administration will still provide the green light of the Modern vaccine, but if possible, the US may begin to receive some 200m pre-ordered doses. The first 20m lottery can be rolled out before December.
Elsewhere
The United States has agreed to purchase 200m jab prices, and 6m will be ready to ship as soon as the vaccine is approved by the FDA.
In Canada, the government plans to receive two million doses of Moderna in March – part of a total of 56m. On Tuesday, Canadian Prime Minister Justin Trudeau said 168,000 doses would be needed before the end of the month.
UK has already pre-ordered 7m doses of Moderna jab.
The European Union last month announced a contract to buy 80m doses – up to 80m more – if the vaccine was considered safe and effective.
Japan has registered 50m Moderna, South Korea 20m, and Switzerland 7.5m, according to data compiled by Duke University Global Health Innovation Center.






Leave a Reply
You must be logged in to post a comment.