 News
News
The All-New Anti-Depressant Nasal Spray
The U.S. Food and Drug Administration today approved Spravato (esketamine) nasal spray, in conjunction with an oral antidepressant, for the treatment of depression in adults who have tried other antidepressant medicines but have not benefited from them (treatment-resistant depression).
What Is It Made Up Of?
The first new class of antidepressant drugs in more than three decades was approved in March when the U.S. Food and Drug Administration (FDA) fast-tracked esketamine, the chemical cousin of illegal street drug ketamine, to prescribe to patients with treatment-resistant depression.
Developed by Johnson & Johnson’s subsidiary, Janssen Pharmaceuticals, esketamine is marketed under the name Spravato and dispensed as a nasal spray administered under the supervision of trained health care professionals.
Ketamine is the chemical mixture of esketamine and arketamine, two mirror-image molecules. But when the molecules are separated, esketamine has been shown to be more potent. As a result, it requires a lower dosage and has a decreased risk of disassociation, tolerance and abuse.
“That’s why Janssen preferred to develop esketamine. It has more robust antidepressant efficacy with less side effects,” said Rodrigo Machado-Vieira, M.D., Ph.D., professor of psychiatry and behavioral sciences and director of the Experimental Therapeutics and Molecular Pathophysiology Program at UTHealth Harris County Psychiatric Center. “It’s the most striking discovery in psychiatry in the past 34 years at least, so I’m very excited.”
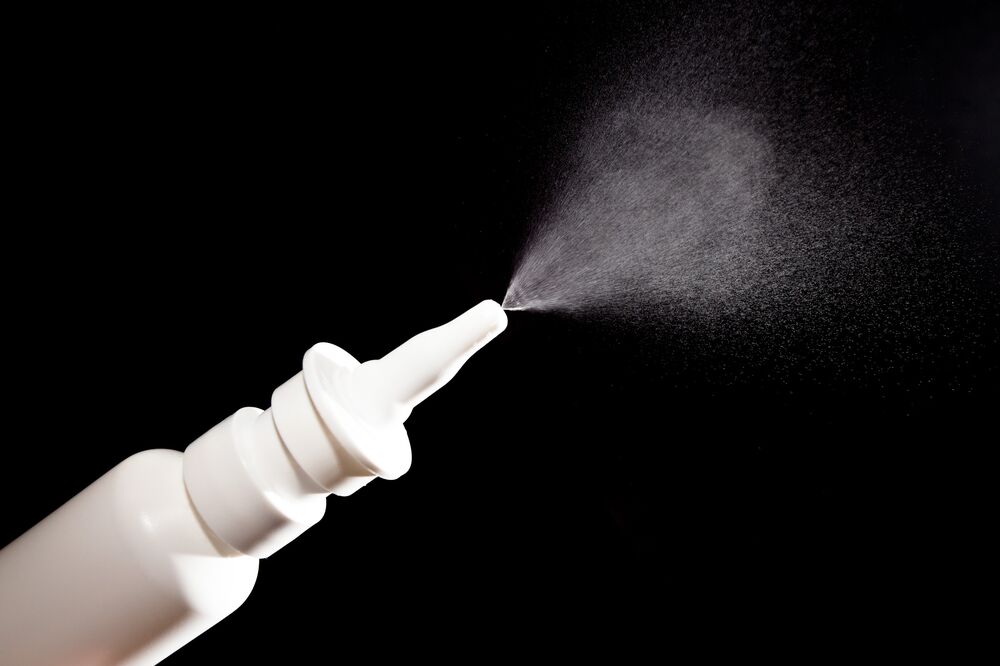
Spravato – The Nasal Spray
Spravato has been used by about 6,000 people for treatment-resistant depression since its approval in March 2019, Kramer said. J&J’s decision to study it in depressed people actively contemplating suicide bucks a trend among drugmakers who routinely exclude such patients from trials.

Part of the thinking behind the decision was that Spravato’s ability to act quickly could mean it works differently than older antidepressants that can take weeks to kick in, Kramer said. In its studies, J&J found those who got the drug had a rapid reduction in the severity of their thinking, although the results didn’t differ in a statistically significant way from patients given a placebo.
The data from studies of the drug shows it “may offer clinicians a new way to provide support to patients quickly in the midst of an urgent depressive episode and help set them on the path to remission,” said Gerard Sanacora, director of Yale’s Depression Research Program and a trial investigator.
The Results Achieved Via Spravato
“Relatively rapidly within a few weeks we saw the numbers stabilize, which was pretty interesting for us and validating in the sense that clinic and patients alike were continuing to make this available,” Kramer said. “We certainly see more and more sites sign on and more and more patients are treated.”
Spravato is a close chemical cousin of the anesthetic ketamine, which differs from existing antidepressants because it acts on the glutamate system in the brain rather than on seratonin or norepinepherine. Scientists have been working to better understand how the drug helps patients and why it works so quickly.
The drug’s approval last year marked the first major breakthrough for depression since 1987. President Donald Trump has since trumpeted the drug as having the potential to curb veteran suicides, but a Veterans Affairs medical panel only approved the drug’s use on a limited basis.
Side-Effects
Patients with unstable or poorly controlled hypertension or pre-existing aneurysmal vascular disorders may be at increased risk for adverse cardiovascular or cerebrovascular effects. Spravato may impair attention, judgment, thinking, reaction speed and motor skills. Patients should not drive or operate machinery until the next day after a restful sleep. Spravato may cause fetal harm and women of reproductive potential should consider pregnancy planning and prevention; women should not breastfeed while being treated.
Esketamine is the s-enantiomer of ketamine. Ketamine is a mixture of two enantiomers (mirror image molecules). This is the first FDA approval of esketamine for any use. The FDA approved ketamine (Ketalar) in 1970.
Suicides In America
America has been in the throes of a suicide crisis even before the pandemic, with the rate rising 30% from 1999 to 2016. Covid-19 closures limited the number of people given the spray as a depression treatment in-person at specified centers.
Ultimately, though, the numbers improved as patients and centers adapted and concerns grew within the mental health community that physical distancing and social isolation of quarantine may exacerbate people’s existing problems or introduce new ones.
Recent Posts
- Bybit Crypto Exchange Hacked, Over $1.4 Billion in Ethereum Stolen in Record-Breaking Breach
- How Meme Coins and Influencers Are Cashing In Like Never Before
- Artificial Intelligence: Revolutionizing Music Production
- Navigating the Complexities of Title IX with Joseph Lento
- This AI-Driven Japanese Company’s Stock Has Soared 400% in 2024
- Jake Paul’s Manager Offers $20mln to IShowSpeed: “Put your signature where your Clout is”
- Is IShowSpeed Going to Be Jake Paul’s Next Boxing Opponent?
- Alessandro Peticchia: An expert in Solar PV and Renewable Energy Projects
- Sony Unveils PS5 Pro: More Power, Higher Price Tag
- New Starbucks CEO Brian Niccol Has a Turnaround Plan! Will it work?


